| |
Vaccination for Seasonal Influenza
|
 |
Influenza Viruses
|
| Influenza is an acute infectious disease caused by the virus family of Orthomyxoviridae. Outbreaks of influenza happen worldwide; and the disease is spread via the respiratory droplets. Patients may manifest symptoms such as malaise, acute febrile illness, runny nose, coughing, headache, muscle aches and sore throat; severe cases may lead to respiratory failure or even death. |
| |
| There are three main types of influenza viruses: A, B and C. Types A and B influenza account for most infections. For most of the years, outbreak of influenza is usually associated with the type A virus; while the type B virus epidemics will tend to occur at intervals of several years. Influenza type C causes mild infection and is regarded not likely to cause epidemics. |
| |
 |
In Hong Kong, influenza is most common during the winter (January to March) and summer (July to August). The H3N2 virus and the H1N1 virus (both type A influenza viruses) epidemics have imposed stress and burden to the community; while the type B influenza virus still keep posing the alarm. |
| |
| The naming and classification system for influenza viruses is based on a World Health Organization (WHO) memorandum. For the type A influenza virus, it is divided into subtypes according to the two antigens on the virus surface: haemagglutinin (H) and neuraminidase (N); and it is where “H” and “N” come from for the H3N2 and the H1N1. For the type B, it is not divided into “H” and “N” subtypes, but there are two important subdivisions which keep circulating to cause epidemics: lineages of B/Yamagata and B/Victoria. |
| |
|
Influenza viruses are constantly changing. For each year, the WHO will convene and discuss the recommendation for the composition of influenza vaccines for next annual season. But the time-line for announcements from the WHO will be different for the Northern Hemisphere and the Southern Hemisphere actually due to the differences in the timing of influenza seasons between these two main regions. The WHO will announce the proposed strains for influenza vaccines to be used in the Northern Hemisphere for the next influenza season, normally in February/March of the year so that vaccines would be available for the next winter influenza season which usually occur at the end of the same year or early next year. Similarly, the WHO will announce the proposed strains for influenza vaccines to be used in the Southern Hemisphere in September of the preceding year so that vaccines would be available for the winter season of the following year (the winter season is around June/July in the Southern Hemisphere).
|
 |
| |
|
A typical WHO recommendation on the influenza season would include strains for the Trivalent vaccine and strains for the Quadrivalent vaccine. For the Trivalent vaccine, the recommendation usually contains two Influenza A virus strains, one H1N1 subtype and one H3N2 subtype, and an Influenza B virus strain. For the Quadrivalent vaccine, an additional Influenza B virus strain will be added. The annual WHO recommendation is based on continuous surveillance and monitoring system worldwide for the influenza virus strains that were spreading around people in different zones and communities, and on the assessment of the effectiveness of vaccines of the previous year.
|
| |
|
After the announcement of strain recommendation, the manufacturers can start preparing for the production of vaccines for the coming influenza season. The recommendation from the WHO is a crucial guidance because it comes into effect after deliberation from experts worldwide for predicting predominant virus strains likely to be circulating in the coming season.
|
|
▲ back to top
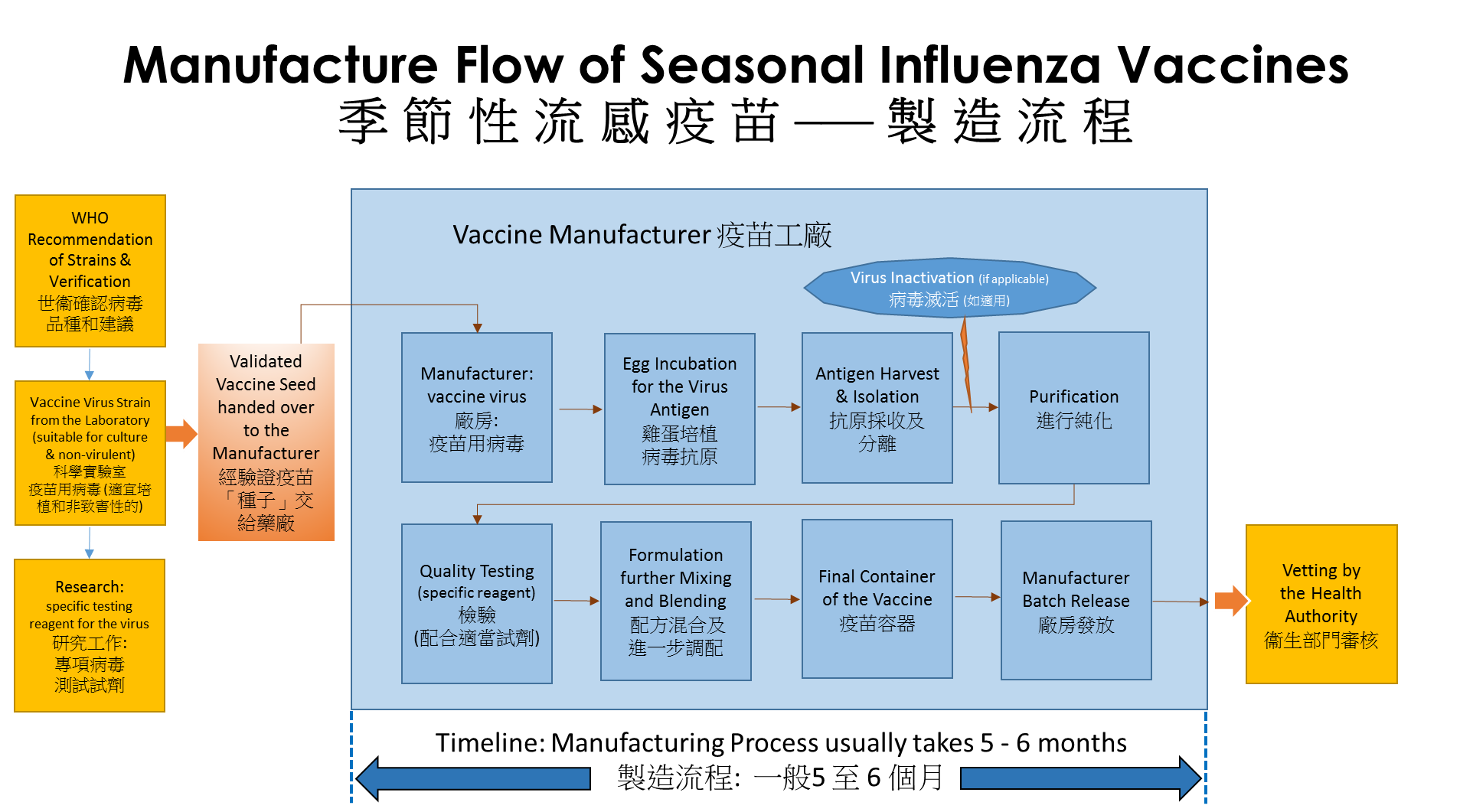
Manufacture of Influenza Vaccines |
 |
Currently, the manufacturing processes for the vaccines generally take at least five to six months before they can be made available on the market, in particular, when a new influenza virus strain is identified. Preparatory works involve the treating of the new influenza virus strain to become a “vaccine virus” which is less dangerous and more readily to grow in hen’s eggs. In additional, the preparatory work also involves developing the reagents to test the vaccine that could take more than 3 months.
|
| |
|
At the vaccine manufacturers, the “vaccine virus” strains are grown under different testing conditions to find the optimal growth conditions in eggs. Eggs are used in the majority of vaccine production, they are fertilized hen’s eggs from flocks under strict hygiene and biosecurity control which meet certain specific requirements.
|
| |
|
During the manufacturing, one strain of the “vaccine virus” as recommended by the WHO is injected into the eggs and incubated for up to 72 hours after which the viruses multiplied in the eggs are then harvested.
|
 |
| |
| The virus molecules are separated from the egg white using a process called ultracentrifugation. Manufacture of the inactivated influenza vaccines will involve the killing/inactivating of the viruses using chemicals, and then purified. Depending on whether a Trivalent vaccine (containing 3 components) or a Quadrivalent vaccine (containing 4 components) is to be manufactured, the incubation of viruses using eggs has to be done up to 3 to 4 times to obtain the required components in the flu vaccine. The bulks of 3 or 4 different flu virus strains will be combined into one bulk and tested for potency, sterility and purity. The bulk will then be diluted to give the desired concentration of antigens which can then be filled into vials or syringes and packaged. |
| |
| After tested to conform potency, sterility and safety standards, regulatory approval is sought before the vaccines could be launched to the market. |
| |
| Other methods of manufacture for seasonal flu vaccines may involve using cultured mammalian cells or recombinant technology. |
| |
|
|
▲ back to top
Constant Updating of Vaccine Strains
|
|
Influenza viruses are quickly changing. Antigens are on the surface of viruses; they are just like “fingerprints” for our human body to recognize and fight against the invading enemy. Antigens are critical for virus strains identification.
|
| |
|
In nature, the principal of virus antigens are susceptible for alteration that results in a somehow a new virus “type” affecting the community. Most of the times, there are only relatively minor changes in the antigen (technically known as antigenic drift). In cases where there is a major change (technically known as antigenic shift), influenza pandemic (worldwide outbreak) is likely to happen. It is the reason why we need constant update of the virus strains in the influenza vaccine.
|
| |

|
Prevention is better than cure. Virus attack is often sudden and aggressive. Our human body need to produce sufficient antibodies (like soldiers) to fight against the virus attack. Vaccination is a preemptive measure to help the body to defend against the versatile viruses. Therefore, it is best to get vaccinated every year for the influenza. Vaccination can provide prior protection; and lower possibility of serious complications and death brought about by real infection.
|
|
▲ back to top
Vaccine Choices
|
|
There are two main types of influenza vaccines: |
|
(a) |
Inactivated Influenza Vaccine (abbreviated: IIV) injection; and |
| (b) |
Live-attenuated Influenza Vaccine (abbreviated: LAIV) nasal spray. |
|
| |
|
The IIV injection (also known as “shot”) is formulated with the viruses that have been inactivated (killed) during the manufacturing process; while the LAIV nasal spray contains viruses that are still alive but already attenuated (i.e. weakened/tamed) right at the beginning of the manufacturing. The attenuated viruses are validated by the medical science that they only trigger restricted growth in the body, and can hardly replicate themselves to manifest into the real illness.
|
| |
|
As opposed to the IIV, the LAIV simulates natural infection through the nose, and may be able to induce stronger and long-lasting immune protection. But on the other hand, because of greater immune response, it could generate more side effects such as runny nose.
|
| |
|
For both IIV and LAIV, the effectiveness will depend on whether the virus strains in the vaccine, based on the WHO recommendation, are compatible with the virus strains that are circulating in the community.
|
|
▲ back to top
Vaccination : Who and When ?
|
|
|
In fact, individuals aged starting from 6 months (i.e. including babies) are recommended to receive the influenza vaccine, especially people having a higher risk of infection and complications such as elderly, children, pregnant women, patients with chronic illness (such as the heart, lung, kidney disease, metabolic disease and immune insufficiency), healthcare providers, poultry workers, pig farmers and other people whose job renders them at higher risk of contracting influenza. Besides, old age home residents, those living in residential care homes for the disabled as well as obese persons (who are fat with Body Mass Index (BMI) value ≥ 30) should also receive the vaccine owing to their high risks to possible influenza complications.
|
 |
| |
|
It is advisable to get vaccinated before the winter influenza season. Sufficient time should also be allowed for the body to boost up the immunity against the influenza viruses, usually taking 2 weeks after vaccination.
|
| |
|
For children aged under 9 years who are receiving influenza vaccine for the very first time, they should receive a second dose; and the doctor will arrange the schedule which is at least 4 weeks after the inaugural dose.
|
| |
Inactivated Influenza Vaccine (IIV)
The IIV is suitable for persons aged 6 months or above.
|
| |
Live-attenuated Influenza Vaccine (LAIV)
The LAIV is another option of the influenza vaccine; it is applicable to persons aged between 2 years and 49 years. However, the LAIV should not be given to individuals, if they have any of the following conditions:
|

|
|
(a) |
Concomitant aspirin or salicylate-containing therapy in children and adolescents; |
| (b) |
Children aged 2 to 4 who are currently having asthma, or have a history of wheezing during the past 12 months; |
| (c) |
Children and adults who are immunocompromised; |
| (d) |
Close contacts and caregivers of severely immunosuppressed persons who require a protected environment; |
| (e) |
Pregnancy; and |
| (f) |
Receipt of influenza antiviral medications within the previous 48 hours. |
|
|
▲ back to top
Precautions and Side Effects for Influenza Vaccines
|
|
People who have previous known history of severe hypersensitivity to the vaccine dose or its components are not eligible. The product leaflets will list the components of specific vaccines.
|
| |
Adverse Effects of the IIV
After receiving vaccination of the IIV, some people may experience the following side effects:
|
| |
|
Nature |
Location |
Description |
Frequency |
|
Mild
|
Injection Site
|
pain
|
very common
|
|
redness, swelling and hardening
|
common
|
|
pruritus
|
uncommon
|
|
Generalized
|
muscle pain, fatigue
|
very common
|
|
fever, headache, sweating, shivering, joint pain, bowel discomfort (e.g. nausea, vomiting, diarrhoea, abdominal pain)
|
common
|
|
dizziness
|
uncommon
|
|
Severe
|
Generalized
|
Anaphylaxis,
Guillain-Barré Syndrome (GBS),
Oculo-respiratory Syndrome (ORS)
|
very rare
|
|
| |
Very common |
(≥1/10 subjects) |
| |
Common |
(≥1/100 to <1/10 subjects) |
| |
Uncommon |
(≥1/1,000 to <1/100 subjects) |
| |
Rare |
(≥1/10,000 to <1/1,000 subjects) |
| |
Very rare |
(<1/10,000 subjects) |
|
| |
| For those who have never received the vaccination before, such as children, they may experience fever, muscle pain and general discomfort. It will usually occur within 6 to 12 hours after injection and last for 1 to 2 days. If fever or discomforts persist, please consult your doctor for follow-up.
|
| |
| Rare and severe manifestations (such as hives, swelling of the lips/tongue and difficulties in breathing) will require emergency medical attention.
|
| |
Adverse Effects of the LAIV
The most common side effects after receiving the LAIV are: runny or stuffy nose for all ages of people; fever in children; and sore throat in adults. They are usually mild in nature and short-lived.
|
| |
|
Nature |
Location |
Description |
Frequency |
|
Mild
|
Localized (nose)
|
runny or stuffy nose
|
very common
|
|
nose bleed
|
uncommon
|
|
Generalized
|
reduced appetite, weakness, irritability, headache
|
very common
|
|
fever, sore throat, cough, muscle pain, chills
|
common
|
|
rash, allergy
|
uncommon
|
|
Severe
|
Generalized
|
severe allergic reactions (e.g. swelling of the face and tongue, shortness of breath),
Guillain-Barré Syndrome
|
very rare
|
|
| |
Very common |
(≥1/10 subjects) |
| |
Common |
(≥1/100 to <1/10 subjects) |
| |
Uncommon |
(≥1/1,000 to <1/100 subjects) |
| |
Rare |
(≥1/10,000 to <1/1,000 subjects) |
| |
Very rare |
(<1/10,000 subjects) |
|
| |
| For the LAIV, children or adolescents aged between 2 years and 17 years should not take any medicines containing aspirin or salicylates, at least 4 weeks after the vaccination, because of the risk of a severe brain disorder called “Reye’s Syndrome”.
|
| |
Allergic Reactions
Egg Protein (Ovalbumin): Manufacture of influenza vaccines from egg-based processes has been adopted for more than 70 years. The advantage of using embryonic eggs to manufacture seasonal flu vaccines is that the safety and effectiveness of the vaccine produced have been well established. But, for those who have egg allergy, it is advisable to talk to their doctors before vaccination. Low levels of residual egg protein could still exist in vaccine that might induce hypersensitivity. Nonetheless, the residual amount is extremely low and is often well tolerated. Inform the doctor if you have previous egg allergy.
|
| |
Thiomersal and Aluminium Salts
Thiomersal, is a mercury-based preservative, which is usually added to multiple dose vaccines. The WHO considers that available evidence strongly supports the use of thiomersal in vaccines and no available evidence suggesting possible health hazard with the amounts of thiomersal currently used in vaccines. However, thiomersal would not be added to vaccines that are intended for single-use only, nor to live-attenuated vaccines. Aluminum salts have been used as an adjuvant in some vaccines. The seasonal influenza vaccines currently marketed in Hong Kong are single-use only which do not contain thiomersal and aluminium.
|

|
|
|
▲ back to top
General advice
|
|
Follow the vaccination schedule as advised by your doctor. It is advisable to receive the vaccine before the influenza season each year.
|
| |
|
To prevent influenza, apart from vaccination, it is best to keep good personal hygiene. Wash or clean hands frequently, especially before touching the mouth, nose or eyes; or after touching surfaces of public installations (such as door-knobs and handrails). Wash hands with liquid soap and water whenever possible and available. When hands are not visibly soiled, cleaning them with 70 - 80% alcohol-based handrub is an effective alternative.
|
 |
| |
|
Maintain good indoor ventilation. When influenza is prevalent, avoid going to crowded or poorly ventilated public places; high-risk individuals may consider putting on surgical masks when staying in such places. Maintain a balanced diet, exercise regularly, take adequate rest, do not smoke and avoid overstress.
|
| |
|
To be considerate for others, cover the nose and mouth when sneezing or coughing and dispose of soiled tissue paper properly in a lidded rubbish bin. Put on a surgical mask when respiratory symptoms develop.
|
| |
|
Aside from influenza, there are also other influenza-like illnesses (ILI) that are transmissible. Remember to seek medical advice for appropriate treatment. More details on influenza and other readings are also accessible via the Centre for Health Protection website.
|
|
▲ back to top
Communication with Your Doctor
|
|
Tell your doctor if you have fever or recently contracted any other infection. If so, it may be advisable to wait until you completely recover from the illnesses.
|
| |

|
In cases, you may be allergic to some substances. Present to your doctor the name of the substance that you are allergic to, so as to know whether you can receive vaccination or not.
|
| |
|
For the IIV injection, discuss with your doctor if you have bleeding problems or are having blood-thinning medications because of the risk of bleeding at injection site on the muscle.
|
| |
|
For the LAIV nasal spray, tell your doctor if you have wheezing recently or have a previous history of asthma; also let the doctor know if you have chronic health problems (such as diabetes, heart, kidney, or lung problems; or a weakened immune system).
|
| |
|
Be cautious if you are concurrently using other medicines (e.g. aspirin, salicylates, or some antiviral drugs). In children (aged between 2 years and 17 years) who are using aspirin or salicylate-containing therapy while receiving the LAIV, there would be a risk for them to develop a serious brain illness called Reye’s Syndrome. On the other hand, if you or your children (of any age) are taking the antiviral drugs (e.g. oseltamivir and zanamivir) while receiving the LAIV, the effectiveness of the LAIV will be impaired; because the antivirals may act against the attenuated viruses in your body for training up the immune response. Therefore, before receiving the vaccination, it is advisable to talk to your doctor for any concurrent medications.
|
| |
|
Tell your doctor if you are pregnant as you will not be eligible for the LAIV.
|
|
▲ back to top
Appearance and Form of Influenza Vaccines
|
|
Vaccines are pharmaceutical products which must be registered before they can be sold or distributed in Hong Kong. Each label must bear a Hong Kong Registration Number (i.e. HK-XXXXX (five numbers)) which can be checked against with the registration record at Drug Office’s website (www.drugoffice.gov.hk). Influenza vaccines are prescription-only-medicines.
|

|
| |
|
The IIV injection and the LAIV nasal spray are the two types of influenza vaccines currently registered in Hong Kong.
|
| |
|
The IIV is presented as liquid suspension used for intramuscular and/or subcutaneous administration; it is usually contained in a single dose prefilled-syringe. For the IIV, both the Trivalent form (with two types of Influenza A and one type of Influenza B viruses) and the Quadrivalent form (with two types of Influenza A and two types of Influenza B viruses) are available.
|
| |
|
For the LAIV, it is presented as liquid suspension for intranasal administration; and is contained in a single dose pre-filled sprayer. In Hong Kong, only the Quadrivalent form of the LAIV is available.
|
|
▲ back to top
Storage and Shelf-life
|
|
Both the IIV and the LAIV have to be stored and transported in refrigerated temperature between 2°C and 8°C.
|
| |
|
The IIV usually have the shelf-life of one year; while the LAIV will have a shelf-life of four months. Any vaccines that have been stored outside the above temperature should not be used. Do not freeze any IIV or LAIV. Discard the vaccines if they have been frozen. Improper storage spoils the vaccine potency.
|
 |
|
▲ back to top
|
|
Acknowledgement : The Drug Office would like to thank the Centre for Health Protection (CHP) and the Professional Development and Quality Assurance (PD&QA) for their valuable contribution to the preparation of this article.
|
|
|
|
|


