| |
Biosimilars: What need to be known?
|
 |
What are Biosimilar Products?
|
| Medications can generally be divided into chemical drugs and biological drugs, with regard to the origin of the material and the manufacturing processes. The chemical drugs will make use of the non-living material and involve chemical synthesis; while the production of biological drugs will make use of living organisms with a variety of processes such as fermentation, culturing cells and manipulating the deoxyribonucleic acid (DNA), etc. |
| |
| Chemical drugs have already predominated in the market for a long time; but with the advances in the biotechnology, “biological” drugs started to gain its place in the past few decades. Examples of some biological drugs include: insulin (from recombinant biotechnology), epoetin (a growth factor for blood components) and antibodies (from “clone” technology). |
| |
 |
New drugs are available, as a result of research and innovation from the manufacturers. Biological drugs can be used to treat or manage diseases, that could not be resolved by conventional chemical drugs. Thus, the biological drugs provide a new source for treating diseases failing traditional drug treatment. However, the prices of these drugs are very high since the innovator is always protected by patent for a number of years (normally 20 years). When the patent is expired, it allows competitors to produce a “copy drug” to the general public. |
| |
| The follow-suit manufacturer makes the “copy drug”. The “copy” for chemical drugs are usually known as “generic” products. |
| |
| The chemical drugs have simpler molecules than the biological drugs. Unlike chemical drugs, the biological drugs have much more sophisticated manufacturing processes; and the biological molecules produced are large and difficult to characterize the nature. The biological drug molecular structure can hardly be made exactly the same as the “innovator” drug. Therefore, the copy version of the biological product, is known as the “similar biotherapeutic product”, or more usually the “biosimilar”. |
|
▲ back to top
Examples of the “Biosimilars” |
| Biological drugs are produced by living organisms. As opposed to conventional chemical drugs, the molecules of biological drugs are very large. (Figure: compare the molecules of the aspirin and the monoclonal antibody) |
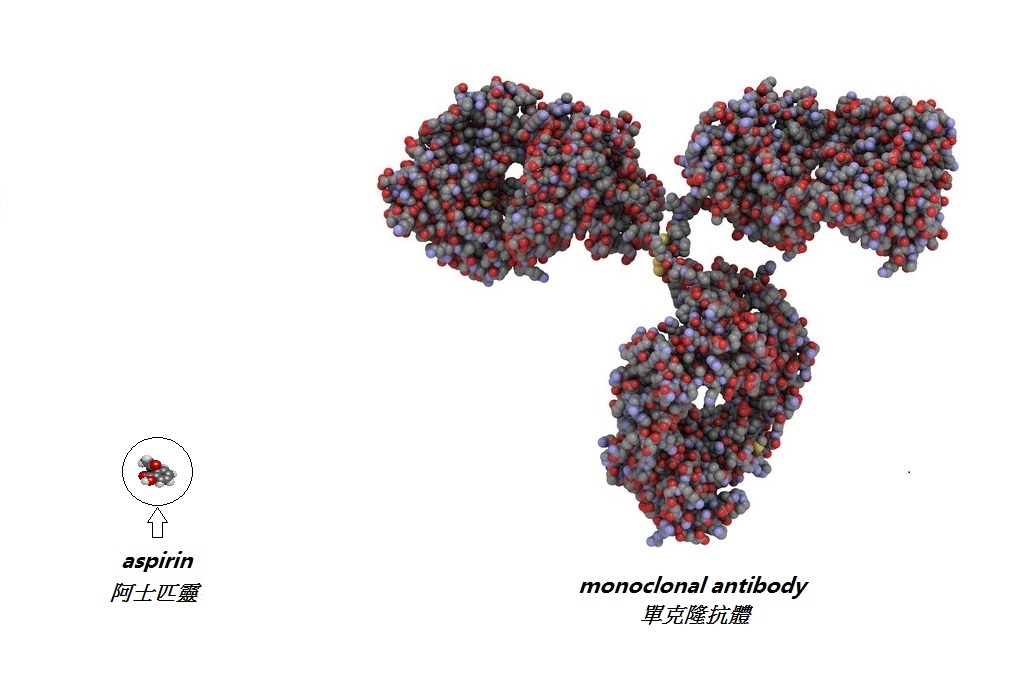
|
| Although it is difficult to copy such a big molecule, some examples of biosimilar drugs have already been manufactured and marketed: |
| |
|
- |
Somatropin: It is a hormone that promotes the bone and muscle to grow. |
| |
| - |
Filgrastim: It is a protein that stimulates the growing of a kind of white blood cell that is essential to our body. It is useful for the patient who has received a cancer treatment or after bone marrow transplant. |
| |
| - |
Infliximab: It is an antibody that helps to inactivate a body substance that is responsible for inflammation. It is useful in patients who have some kinds of diseases caused by immunological dysregulation, such as rheumatoid arthritis and psoriasis. |
|
|
▲ back to top
“Biosimilars” versus “Innovated” Biological Drugs
|
|
The biosimilar product is the copy version of an innovated biological drug.
|
| |
 |
The innovator makes research effort starting from scratch. It has to identify all relevant materials, do validating tests for all necessary processes, and perform studies (including pre-clinical and clinical) that are crucial for the product to be “first” approved into the market. |
| |
|
The follow-suit manufacturer saves the developing costs because it can learn some experience for making the drug; and does not have to “redo” the tedious research work in which the innovator has already invested much time, money and effort.
|
| |
| Nevertheless, making a copy for a biological product requires significant expertise and quality assurance. The production raw materials are highly specific (e.g. the adopted pedigree of cells is unique) and its process is sensitive to tiny changes in conditions, such as temperature, formulation and container material. Any slight changes in the raw materials and the production conditions can lead to considerable variation in the final product. Therefore, to ensure uniformity for the biological product (either the originator or the biosimilar), stringent quality control needs to be in place. |
| |
|
In fact, even in the innovator’s manufactory, and with strict adherence to the production specifications, natural variability exists for the biological products. The innovator will usually develop “standard” tests, to ensure the final product manufactured will have the significant properties that are essential to exert the effect. The tiny variation in the product itself is technically known as “microheterogeneity”.
|
 |
| |
| Therefore, the goal of the follow-suit manufacturer is to minimize the differences between “biosimilar” and “innovator”. It will take reference from the originator’s production and testing methods, in order to make a product to be highly “similar” to the innovator. |
| |
| Comparative studies (sensitive tests in animals and human) are usually available to detect any slight differences between the innovator’s drug and the “biosimilar” product. In other words, it is to rule out any significant differences between the two products for the clinical efficacy and safety profile. |
| |
| Sometimes, a reference originator product may have more than one indication (indication means: the disease or symptom that is labelled to be treatable and applicable by a medication). In that case, if the comparative clinical studies (tests in human) are able to prove that the biosimilar is “similar” to the reference product for treating the “studied” indication, it can be expected that (on a case-by-case basis) the biosimilar is also allowed for the other indications that the reference product has already been approved. The extension of indications for biosimilar product is technically known as “extrapolation”. |
|
|
▲ back to top
The Aspects of Interchangeability
|
|
Then, the question is: Can the “biosimilar” product be switched with the innovator product? |
| |
|
Each biological product (notwithstanding the originator or the biosimilar) has its “unique” profile, they could not be regarded as the “same” with each other.
|
| |
 |
In the aspect of dispensing, in case where the doctor prescribes a particular brand, the pharmacist is not allowed to substitute the prescribed brand with another brand of medicine, unless having consensus with the prescribing doctor.
|
| |
|
When a patient has started treatment on one particular brand of biological drug, it is not advisable to switch to another “brand” unless the switching is clinically justifiable and having discussed with the patient. Should there be any uncertainty, it is recommended to consult the “first-prescribing” doctor, and with any potential risks adequately assessed.
|
|
▲ back to top
General Advice and Precautions
|
 |
Biological products (including biosimilar) are produced by the living organism; and their molecules are usually protein in nature with complex structure. Each biological product will have its own risk profile for the potential to trigger an immune response; and the specific risk has been assessed before launching to the market. Usually, there could only be little or no immune response at all; and immune reactions (e.g. infusion-related reactions or injection site reactions), if happened, are normally transient and not severe in nature. But there may be situation that our immune system (e.g. the antibodies) will neutralize the biological drug and thereby reducing the drug efficacy. And there could be “rare immune reaction” against the drug that may be serious and life-threatening.
|
| |
|
Therefore, doctors need to monitor any adverse effects on the biological drug treatment; while patients should inform health care professionals for the side effects.
|
|
▲ back to top
Communication with your Doctor
|
|
Take advice from your doctor for the treatment choice. The doctors will rely on their professional judgements to prescribe the biological products (e.g. the originator product or biosimilar products). During treatment, your doctor may inform you if there would be any brand switching or changes, when necessary.
|
 |
| |
|
Your doctor may ask you to be alert of some specific side effects. In case you experience any of them, do not hesitate to inform your doctor immediately about your symptoms, especially for those related to immune system, e.g. allergy or inflammation.
|
|
▲ back to top
Labelling of the Biosimilar Product
|
 |
Biosimilar products, like all other pharmaceutical products in Hong Kong, need to be registered before they can be sold or distributed in Hong Kong. Each label must bear a Hong Kong Registration Number (i.e. HK-XXXXX (five numbers)) which can be checked against with the registration record at the Drug Office’s website (www.drugoffice.gov.hk).
|
| |
|
On the label of a registered biosimilar product, you may be able to find:
|
| |
|
- |
a statement indicating that it is a biosimilar product; |
| |
| - |
the brand name of the product, and the name of the active substance; and |
| |
| - |
the approved indications and brief descriptions on related clinical studies. |
|
|
▲ back to top
Storage Condition for Products
|
|
Biological products (including biosimilars) are usually sensitive to heat and susceptible to microbial contamination. Most biological products come in as injectables and they should normally be stored between 2°C and 8°C and kept away from light. In general, it is advisable to check the label of each product for the specific storage instructions.
|
|
▲ back to top
|
|
Acknowledgement: The Drug Office would like to thank the Professional Development and Quality Assurance (PD&QA) and the Social Hygiene Service (SHS) for their valuable contribution to the preparation of this article.
|
|
|
|
|


