General Information |
-
Q.1What is considered as a “pharmaceutical product” or “medicine” in Hong Kong?
A.1 Under the Pharmacy and Poisons Ordinance, “Pharmaceutical product” or “medicine” —
-
Q.2 What is the difference in the control between Western medicine and Chinese medicine in Hong Kong?
A.2 In general, Western medicine refers to pharmaceutical product as defined above in A.1 and is controlled under the Pharmacy and Poisons Ordinance (Cap. 138). For Chinese medicine, it is controlled under Chinese Medicine Ordinance (Cap. 549). The sale, manufacturing, dispensing or compounding of Chinese herbal medicines or proprietary Chinese medicines as defined in section 2 of the Chinese Medicine Ordinance (Cap 549) or other materials of herbal, animal or mineral origin customarily used by the Chinese for medicinal purpose is exempted from regulation under the Pharmacy and Poisons Ordinance (Cap. 138).
You may find more information on the regulation of Chinese medicines on the Chinese Medicine Council’s website (www.cmchk.org.hk)or the website of the Chinese Medicine Regulatory Office of Department of Health (www.cmro.gov.hk). You may also call the Chinese Medicine Regulatory Office of Department of Health (Tel.: 2319 5119) for enquiries relating to Chinese medicines.
-
Q.3 How can I know more about the legal requirements of handling pharmaceutical products in Hong Kong?
A.3The regulation of pharmaceutical products are mainly provided under the following Laws of Hong Kong:
- Pharmacy and Poisons Ordinance (Cap. 138)
- Antibiotics Ordinance (Cap. 137)
- Dangerous Drugs Ordinance (Cap. 134)
- Undesirable Medical Advertisements Ordinance (Cap. 231)
- Public Health and Municipal Services Ordinance (Cap. 132)
- Import and Export Ordinance (Cap. 60)
The printed versions of these Laws of Hong Kong can be purchased from the Publications Sales Unit of Information Services Department (Tel.: 2537 1910) or from the Government Bookstore ( www.bookstore.gov.hk). The contents of the relevant legislation may also be found on the website of the Bilingual Laws Information System of Department of Justice at:
https://www.elegislation.gov.hk/index/chapternumber?p0=1&TYPE=1&TYPE=2&TYPE=3&_lang=en
-
Q.4Are all pharmaceutical products sold in Hong Kong registered?
A.4As stipulated under Reg.36(1) of the Pharmacy and Poisons Regulations (Cap. 138A), "pharmaceutical products" must be registered before they can be sold, offered for sale, distributed or possessed for the purposes of sales, distribution or other use in Hong Kong.
Sale of unregistered pharmaceutical products is an offence under the Pharmacy and Poisons Ordinance. The maximum penalty is a fine at level 6 of Criminal Procedure Ordinance Cap 221 ($100,000) and two years' imprisonment.
-
Q.5 Once registered, can all pharmaceutical products be freely sold in Hong Kong?
A.5Registered pharmaceutical products are subject to various kinds of control over their sale to protect the health of the public. In Hong Kong, the Poisons List under the Tenth Schedule of Pharmacy and Poisons Regulation (Cap. 138A) lists out those ingredients classified as poisons. Some poisons are further categorized under different Parts of the Poisons List and other different Schedules under the Pharmacy and Poisons Regulations (Cap. 138A) according to their potency, toxicity and potential side-effects.
Such categorization determines the different levels of control over their sale. For example, pharmaceutical products that do not contain any poisons or contain Part 2 poisons are referred as Over-The-Counter medicines (OTC). The former can be sold in any retail shops while the latter can be sold in Authorized Sellers of Poisons (ASP, usually known as pharmacies or dispensaries) and Listed Sellers of Poisons (LSP, usually known as medicine stores). Pharmaceutical products containing Part 1 poisons can only be sold in pharmacies (ASP) in the presence and under the supervision of registered pharmacist.
Some Part 1 poisons are further classified into First Schedule and the Third Schedule with additional restrictions on their sale at the retailers. The sale of pharmaceutical products containing Part 1 First Schedule poisons further requires keeping sales records which include the date of the sale, the name, number of identity card, address and signature of the purchaser, the name and quantity of the medicine as well as the purpose for which it is required. The sale of pharmaceutical products containing prescription only medicines (Part 1 Third Schedule poisons) must be authorized by a prescription from a registered medical practitioner, a registered dentist or a registered veterinary surgeon.
Antibiotics defined under the Antibiotics Ordinance (Cap. 137) and dangerous drugs defined under the Dangerous Drugs Ordinance (Cap. 134) are also prescription only medicines.
The sales requirement of various categories of pharmaceutical products is summarized in the Appendix I.
-
Q.6 Why do the outer boxes of some pharmaceutical products bear the word“Prescription Drug 处方药物” or “Drug under Supervised Sales 监督售卖药物”?
A.6According to the Pharmacy and Poisons Ordinance, all Part 1 poisons and Part 2 poisons must be labelled with the word "Poison 毒药" or other applicable words specified in the above Ordinance. The word "Poison 毒药" was used to serve as a warning to consumers as improper use of these medicines may cause serious health damage in the past. These medicines should only be used upon advice from healthcare professionals.
With effect from 5 August 2016, pharmaceutical products or medicines containing Part 1 poisons (except those included in the Third Schedule) should be labelled with the words "Drug under Supervised Sales 监督售卖药物"; pharmaceutical products or medicines containing poisons included in the Third Schedule should be labelled with the words "Prescription Drug 处方药物".
-
Q.7How can I check whether the pharmaceutical product I am buying is registered or not?
A.7The Hong Kong registration number for a registered pharmaceutical product should be labelled on the sales pack in the form of "HK-XXXXX" as seen in the example in A.22 (Appendix II).
The information of all registered pharmaceutical products can be identified from:
(1) Drug Office’s website: By entering the pharmaceutical product’s particulars such as its English product name or its Hong Kong registration number, information can be obtained through the “Search Drug Database” at the following link:
http://www.drugoffice.gov.hk/eps/do/en/consumer/search_drug_database.html
or by viewing the Compendium of Pharmaceutical Products in form of PDF format at the following link:
http://www.drugoffice.gov.hk/eps/do/en/doc/Compdium.pdf
(2) Compendium of Pharmaceutical Products: The Compendium (in CD-ROM format) is freely distributed to the public at the following locations of Drug Office :-
(i) DE&IE Division - Suites 2002-05, 20/F, AIA Kowloon Tower, Landmark East, 100 How Ming street, Kwun Tong, Kowloon, Hong Kong
(ii) RM&O Division - Room 1801, 18/F, Wu Chung House, 213 Queen's Road East, Wanchai, Hong Kong
(iii) Administrative Division - Room 1856, Wu Chung House, 213 Queen's Road East, Wanchai, Hong Kong
(iv) L&C Division - Room 2550, 25/F, Wu Chung House, 213 Queen's Road East, Wanchai, Hong Kong
(v) L&C Division - Room 2001-2002, 20/F, Dah Sing Financial Centre, 248 Queen's Road East, Wanchai, Hong Kong
-
Q.8 Why does the active ingredient printed on the outer boxes of certain pharmaceutical products have more than one common name?
A.8In general, only one name of each type of active ingredient shall be printed on the label. However, if the names amongst the Poisons List of the Pharmacy and Poisons Regulations (Cap. 138A), the International Nonproprietary Names (INN) published by World Health Organisation, or other synonyms commonly used in other countries/areas are different, more than one common name may be printed on the label of pharmaceutical products for easy identification and searching relevant information on this website.
-
Q.9 Are all registered pharmaceutical products available in the Hong Kong market?
A.9A pharmaceutical product that is registered in Hong Kong does not necessarily mean that it is locally available. The decision on whether to market a pharmaceutical product in Hong Kong is made by its registration certificate holder.
To find out whether a product is available in Hong Kong, you can seek advice from a local pharmacy (ASP) or medicine store (LSP) or you can contact the registration certificate holder. Information on the registration certificate holder of a pharmaceutical product can be found from the “Search Drug Database” of Drug Office’s website at the following link:
http://www.drugoffice.gov.hk/eps/do/en/consumer/search_drug_database.html
-
Q.10 As registered pharmaceutical products are regulated by the relevant authority, does it mean that they will not cause harm to consumers?
A.10A pharmaceutical product or medicine must meet the criteria of safety, quality and efficacy for approval of registration. But no medicine is risk free. Prior to a product’s registration, information is only available from pre-market studies of the product and reference from reputable literatures. In addition, some adverse effects of a medicine will only be noted after the product is marketed and used in the general population. Therefore, assessment of a product prior to its registration can only be based on information available at that time.
Furthermore, medicines may cause adverse effects and response that vary amongst individuals. You should seek healthcare professionals’ advice on whether a medicine is appropriate for your health condition or management of your disease.
-
Q.11 How can my health be protected if my medicine causes side effects?
A.11Healthcare professionals will balance the risks and benefits of a particular treatment for their patients according to their conditions. If a medicine is used in accordance with the advice from your doctor or pharmacist, the health benefits you gain from the treatment should outweigh the risks of adverse events. Some risks of a medicine will not be revealed until after the drug has been used in larger populations and in different types of patients. You are therefore encouraged to report to a healthcare professional any adverse effects you experience after taking a medicine. Healthcare professionals will then report any adverse drug reactions that deemed medically significant to our office. Such data is useful for monitoring the safety of registered pharmaceutical products.
▲ Back to top
-
-
Buying and selling medicines
-
Q.12 Is it safe to purchase pharmaceutical product from the Internet?
A.12According to the information from World Health Organization, over 50% of the medicines purchased via the Internet from sites that conceal their physical address have been found to be counterfeit. In addition, improper storage of a pharmaceutical product during transit might adversely affect the quality of the product.
You are, therefore, advised not to purchase pharmaceutical product from sources that are doubtful, including from Internet sites with unknown origin or seller with unknown address, as the product’s safety, quality and efficacy cannot be guaranteed.
-
Q.13 What is the harm of self-medication?
A.13Self-medication may pose risk to you in many ways, such as:
- Failing to recognize the symptoms of serious underlying diseases and delay appropriate treatment;
- Failing to select an appropriate product or use the correct dosage for your medical conditions; and
- Failing to monitor the response to the treatment and its possible adverse effects.
You are advised to seek advice from healthcare professionals before using any medicine instead of choosing one by yourself, especially if you have persistent symptoms of unknown cause. They can give you the most appropriate treatment option after considering your conditions as well as the risks and benefits of the medicine.
-
Q.14 If I have some extra medicines, will it be alright to sell them over the Internet?
A.14In Hong Kong, pharmaceutical products must be registered before sale and it is unlawful to sell unregistered pharmaceutical products. Any illegal sale of unregistered pharmaceutical products will contravene the Pharmacy and Poisons Ordinance (Cap.138) and the maximum penalty is a fine at level 6 of Criminal Procedure Ordinance Cap 221 ($100,000) and imprisonment for 2 years upon conviction.
In addition, even if the product is registered, their sale may be subject to different levels of control (please refer to A.5) and you may contravene the relevant legislation in dealing with or trading pharmaceutical products without the appropriate licences or authorisation.
-
Q.15 I am taking some medicines which are prescribed by my doctor in my hometown and I have to stay in Hong Kong for a period of time. How can I obtain these medicines in Hong Kong?
A.15You can check whether your medicines are registered in Hong Kong by the method suggested in A.7. You should also discuss with a local healthcare professional, such as a doctor or pharmacist to check whether you can obtain the same medicines in Hong Kong or to advise to switch to another substitute if they are not available.
-
Q.16 Can I buy some pharmaceutical products overseas for my own use and bring them back to Hong Kong?
A.16Importation of pharmaceutical products and medicine are controlled under the Import and Export Ordinance (Cap. 60), thus must be covered by a licence issued by Department of Health under delegated authority of the Director-General of Trade and Industry Department. The application of these licences should be submitted to the Drug Evaluation and Import/Export Control Division of the Drug Office of Department of Health.
Pharmaceutical products and medicines imported in the personal baggage of a person entering Hong Kong and which are accompanied by him and in a reasonable quantity for his personal use may be exempted from licensing requirement.
You may also call the Drug Office of Department of Health (Tel.: 3974 4180) for enquiries relating to import/export of pharmaceutical products and medicine or Customs and Excise Department (Tel.: 2815 7711) for general enquiry on Customs clearance.
-
Q.17 Other than in situation specified in A.16, can I import/ export pharmaceutical products for personal use by post or any other means ?
A.17Department of Health does not issue import/export licences to members of public who wish to import/ export pharmaceutical products for personal use by post or any other means.
If you are a resident in Hong Kong and need medicines for medical treatment, you should consult local medical practitioner who should be able to provide appropriate medicines for your conditions, or to advise to switch to another substitute if they are not available.
-
Q.18 Why can’t I sell these pharmaceutical products that I bought abroad in Hong Kong, even over the Internet?
A.18It is a legal requirement that pharmaceutical product must be registered before sale in Hong Kong. By the registration system, the product’s safety, quality and efficacy can be properly evaluated so as to safeguard public health. Also, registered pharmaceutical products should bear the proper labeling requirement including registration number (i.e. HK-XXXXX) in order to provide sufficient information to the general public.
-
Q.19Tell me more about the recent changes in the sales control of codeine-containing medicines.
A.19Starting from 26 January 2024, it is a legal requirement for pharmacy to record the purchaser's personal information, including Identity Card number, when supplying codeine-containing medicines without doctor's prescription.
The Government had always been concerned about the abuse of codeine containing medicines, the Pharmacy and Poisons Board of Hong Kong reviewed the regulatory control of codeine-containing medicines and decided to strengthen the sales control of non-prescription codeine-containing medicines. With effect from 26 January 2024, all medicines containing less than 0.2% of codeine are regulated as Part 1 Schedule 1 poisons under the Pharmacy and Poisons Regulations (Cap. 138A).
In other words, when supplying a codeine-containing medicines, an Authorized Seller of Poisons ('pharmacy' or 'dispensary') must make an entry in the book (known as "poisons book") with particulars of the purchaser, including his/her name, address and Identity Card number. The purchaser is also required to sign in the poisons book accordingly. This requirement only applies to non-prescription codeine-containing medicines, i.e. medicines containing less than 0.2% codeine.
▲ Back to top
-
Medicine shops
-
Q.20Why some shops selling medicines have a “Rx” logo displayed outside the shops?
A.20In Hong Kong, the sale of pharmaceutical products are subject to different levels of control (please refer to A.5). Only an Authorized Seller of Poisons (ASP), often referred to as a “pharmacy”, can sell prescription medicines under the authority of a prescription with the supervision of a pharmacist. At these premises, a pharmacist will be on duty at specified hours and it is allowed to use or display the “Rx” logo.
-
Q.21I see quite a lot of “Medicine Stores” without the display of “Rx” logo. Are these premises allowed to sell medicines?
A.21Listed Seller of Poisons (LSP), or referred to as “medicine stores” are different from pharmacies (ASP) in that there are no pharmacists on duty in the former. They cannot sell pharmaceutical products containing Part 1 poisons, including prescription medicines, and are prohibited from using or displaying the “Rx” logo. However, they are authorized to sell medicines that are non-poisons or Part 2 poisons, such as cold and flu medications.
-
Q.22How can I verify whether a shop is a registered pharmacy?
A.22To check whether a shop is a registered pharmacy (ASP), you can verify the information from “List of Authorized Seller of Poisons” of Drug Office’s website at the following link:
http://www.drugoffice.gov.hk/eps/do/en/consumer/news_informations/relicList.html?indextype=4A
Furthermore, a registered pharmacy must have a pharmacist on duty. The certificate of registration of the duty pharmacist and his/her attendance hours should be displayed at a conspicuous space in the shop. Some pharmacies may display the “Rx” logo.
-
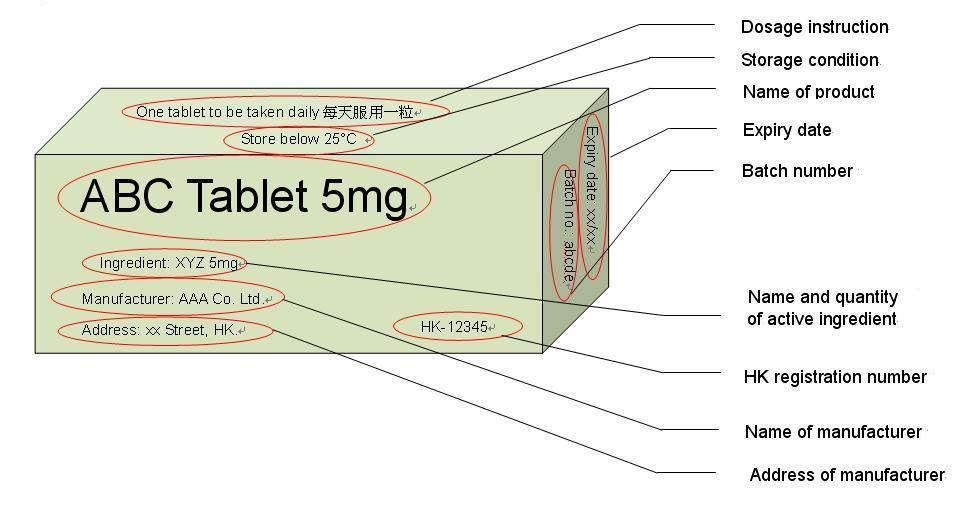
Q.23When I buy medicines from a medicine store, what information should I pay attention to?
A.23Medicine stores (LSP) are authorized to sell medicines that are non-poisons or Part 2 poisons, such as cold and flu medications. For these registered products, you should be able to find the following information on the label/outer box, as shown in the example:
- Product Name
- HK registration number (HK-XXXXX)
- Name and quantity of active ingredient
- Expiry date
- Batch number
- Storage condition
- Name and address of the manufacturer
- The dosage instructions and cautionary statement (if any) should be labelled in both English and Chinese.
You can refer to the sample box in Appendix II.
-
Q.24My friend recommended a medicine to me and I have the name of the product. Can I purchase it from a pharmacy or medicine store?
A.24Products with very similar names or appearance may contain different ingredients while products with the same ingredients may have different product names or appearance. Therefore, self-medications may lead to overdose or increase the risks of side effects, posing significant risk to your health. You should always seek advice from a qualified healthcare professional before taking any medicines.
▲ Back to top
Prescription
-
Q.25If I want to buy a medicine, do I always need to have a prescription?
A.25In Hong Kong, the sale of pharmaceutical products are subject to different levels of control (please refer to A.5) and those used for the treatment of diseases that require diagnosis and management by doctors are usually prescription-only medicines. Examples include medicines used for the treatment of high blood pressure, diabetes, bacterial infections and medicines that help you to sleep. These prescription-only medicines must be dispensed and sold on doctor’s prescription in registered pharmacies (ASP) under the direct supervision of registered pharmacists.
On the other hand, medicines with fewer serious side effects or those used for treating or alleviating minor illness can be sold without prescription in pharmacies (ASP) or medicine stores (LSP). Examples include drugs used for relieving common cold, treating fever and some painkillers. You may consult pharmacists for more information.
-
Q.26What information should be included in a valid prescription?
A.26A prescription should be given by a registered medical practitioner, registered dentist or registered veterinary surgeon. In the prescription provided by a dentist, it should be written “For dental treatment only” and in the prescription given by a veterinary surgeon, it should be written “For animal treatment only”. Other information that should be included in a valid prescription are:
- Date of the prescription
- Name and address of the patient
- The total amount of the medicine to be supplied and the dose to be taken or administered
- Signature of the prescriber
- Address of the prescriber
-
Q.27Where should I go to fill my prescription?
A.27You should bring your prescription to a registered pharmacy (ASP) for dispensing. You can find information of registered pharmacies at the “List of Authorized Seller of Poisons” of Drug Office’s website at the following link:
http://www.drugoffice.gov.hk/eps/do/en/consumer/news_informations/relicList.html?indextype=4A
-
Q.28Can I keep my prescription after filling it?
A.28It is a legal requirement for pharmacies (ASP) to retain prescriptions dispensed and keep the records for 2 years. The pharmacist in the shop has to maintain prescription records which should be readily available for inspection. If you want to have your own record, you can make a photocopy before filling it.
-
Q.29Can a prescription be dispensed more than once?
A.29The person dispensing the prescription shall not dispense the prescription more than once unless the prescriber has directed on the prescription that -
(1) it may be dispensed a stated number of times; or
(2) it may be dispensed at stated intervals
At the time of dispensing a prescription which can be dispensed more than once, the name and address of the pharmacy (ASP) and the date on which the prescription is dispensed should be noted on the prescription by the person dispensing the prescription. The prescription will be retained by the pharmacy which carried out the last and final dispensing of the prescription.
▲ Back to top
Others
-
Q.30What should I do if I feel unwell after taking medicine?
A.30As the responses to a medicine may vary amongst individuals, you should tell the doctor or pharmacist who give you the medicine about your symptoms after taking medicine.
Certain people may be allergic to a medicine and develop symptoms like rash or urticaria. In more severe cases, one may experience reactions like shortness of breath and in such case, you should seek medical treatment immediately.
You should tell your doctor or pharmacist any of your known drug allergies as there may be cross-sensitivity among drugs in the same class.
-
Q.31Can I call Drug office for advice on my current treatment or medical condition?
A.31Decision on the treatment regime for a patient needs professional evaluation of patient’s symptoms, signs, medical history, and their response to medications.
If you have query concerning your treatment or medical condition, you should discuss with healthcare professionals such as your family doctor or pharmacist. The Drug Office is a drug regulatory agency and is not able to advise individual patients on their treatment. However, you can contact us (Tel.: 2572 2068) for any complaint on a pharmaceutical product.
-
Q.32If I want to raise a complaint about a product marketed with a Hong Kong registration number, what should I do?
A.32If the product you have on hand is labelled with the registration number indicated as HK-XXXXX, you can contact us (Tel.:2572 2068) for any complaint on the pharmaceutical product. You can also send us an email at pharmgeneral@dh.gov.hk with details of your complaint.
However, if the product’s registration number is indicated as HKP-XXXXX, HKNT-XXXXX or HKC-XXXXX, it is a proprietary Chinese medicine. You should contact the Chinese Medicine Regulatory Office of Department of Health (Tel.: 2319 5119).
-
Q.33If I suspect that a shop is selling counterfeit medicine, what should I do?
A.33You are encouraged to report suspected counterfeiting activities to the Customs and Excise Department by :
- Customs Hotline (24-hour)
(852) 2545 6182
- Fax
(852) 2543 4942; or
- Mail
Commissioner of Customs and Excise
G.P.O Box No. 1166
-
Q.34How to give medicine to children?
A.34
Parents and caregivers should follow the instructions for use for all medicines given to children. The package insert and label should be read carefully and suggesting that attention should be given particularly to the dose, frequency, route of administration and special conditions of the medicines before giving them to children. The outer box of the medicine should be maintained for knowing which active ingredients are taken by children, and for checking if another medication containing same ingredients is taking at the same time to avoid drug overdose.
Children should be supervised when taking any dose form of medicine; particular attention should be paid for taking soft gel capsules due to the risk of choking. Although soft gel capsules are not necessarily unsafe products, and the choking risk posed by these capsules is similar to that for many food items, parents and caregivers should be mindful of the risk of choking when medicines in the form of soft gel capsules are given to younger children.
Proper storage of medicines is not only to maintain the potency of medicine, but also to protect children from accidental ingestion. Medicines should be kept in a cool and dry place where is out of reach of children. Unless specified on the label, medicines should not be stored in refrigerators. The expiry date of medicines should be checked regularly as the expired medicines may cause harm to the health of your children and lose their effectiveness. If you have any further questions on giving medicine to children, consult your doctor or your pharmacist.
For more details on handling medicines safely, please refer to the following website:
http://www.drugoffice.gov.hk/eps/do/en/consumer/news_informations/knowledge_on_medicines/handling_medicines_safety.html
-
Q.35 Does the Drug Office provide medicine testing service to public?
A.35 The Drug Office is a law enforcement agency over the legislations concerning medicines.
In general, the Drug Office does not provide testing service to public. If you wish to test a medicine, you may approach the private laboratories.
The Hong Kong Accreditation Service (HKAS) of the Innovation and Technology Commission provides accreditation to laboratories located in Hong Kong through the Hong Kong Laboratory Accreditation Scheme (HOKLAS). You may wish to refer to the website of HKAS with list of accredited laboratories: https://www.itc.gov.hk/en/quality/hkas/index.html
▲ Back to top
Appendix I
(Appendix of A.5)
| |
Category of medicine |
Sales requirements |
Examples of medicines |
Related laws |
| 1. |
Non-poison |
Medicine can be sold in any retail shop as long as it is registered |
Oral mucolytic drug containing acetylcysteine |
Pharmacy and Poisons Ordinance, Cap. 138 |
| 2. |
Part 2 poison |
Medicine can be sold in a pharmacy (ASP) or a medicine store (LSP) |
Cold and flu medication containing chlorpheniramine |
Pharmacy and Poisons Ordinance, Cap. 138 |
| 3. |
Part 1 poison |
Medicine can be sold in a pharmacy (ASP) in the presence and under the supervision of a registered pharmacist |
Ibuprofen (a non-steroidal anti-inflammatory pain killer) |
Pharmacy and Poisons Ordinance, Cap. 138 |
| 4. |
Part 1 First Schedule poison |
The requirements as item 3 above plus record of the transaction in the poisons book |
Cough syrup containing less than 0.2% of codeine |
Pharmacy and Poisons Ordinance, Cap. 138 |
| 5. |
Part 1 Third Schedule poison |
The requirements as item 4 above plus medicine must be sold on or in accordance with a prescription given by a registered medical practitioner, registered dentist or a registered veterinary surgeon |
Anti-hypertensive drug (e.g. amlodipine, metoprolol), Anti-diabetic drug (e.g. gliclazide, metformin), Oral corticosteroid drug (e.g. dexamethasone, prednisolone, prednisone), Some non-steroidal anti-inflammatory drugs (e.g. indomethacin, mefenamic acid) |
Pharmacy and Poisons Ordinance, Cap. 138 |
| 6. |
Antibiotic |
Amoxycillin, cephalexin, erythromycin |
Antibiotics Ordinance, Cap. 137 |
| 7. |
Dangerous drug |
Diazepam, phentermine |
Dangerous Drugs Ordinance, Cap 134 |
Appendix II
(Appendix of A.22)
26 January 2024
|
|



